Large Unmet Clinical Needs in Stroke, Traumatic Brain Injury (TBI) and Age-related Macular Degeneration (AMD)

Investigational medical device not cleared for human use
As there are no approved TBI therapies, the current standard is 'rest.' Repeated concussions can lead to cognitive impairment and other neurodegenerative diseases.
With nearly 4 million annual cases of TBI in the USA, our novel approach, intranasal delivery of elovanoids, addresses a large unmet clinical need.
Ischemic Stroke
Traumatic Brain Injury (TBI)
Age-related Macular Degeneration (AMD)

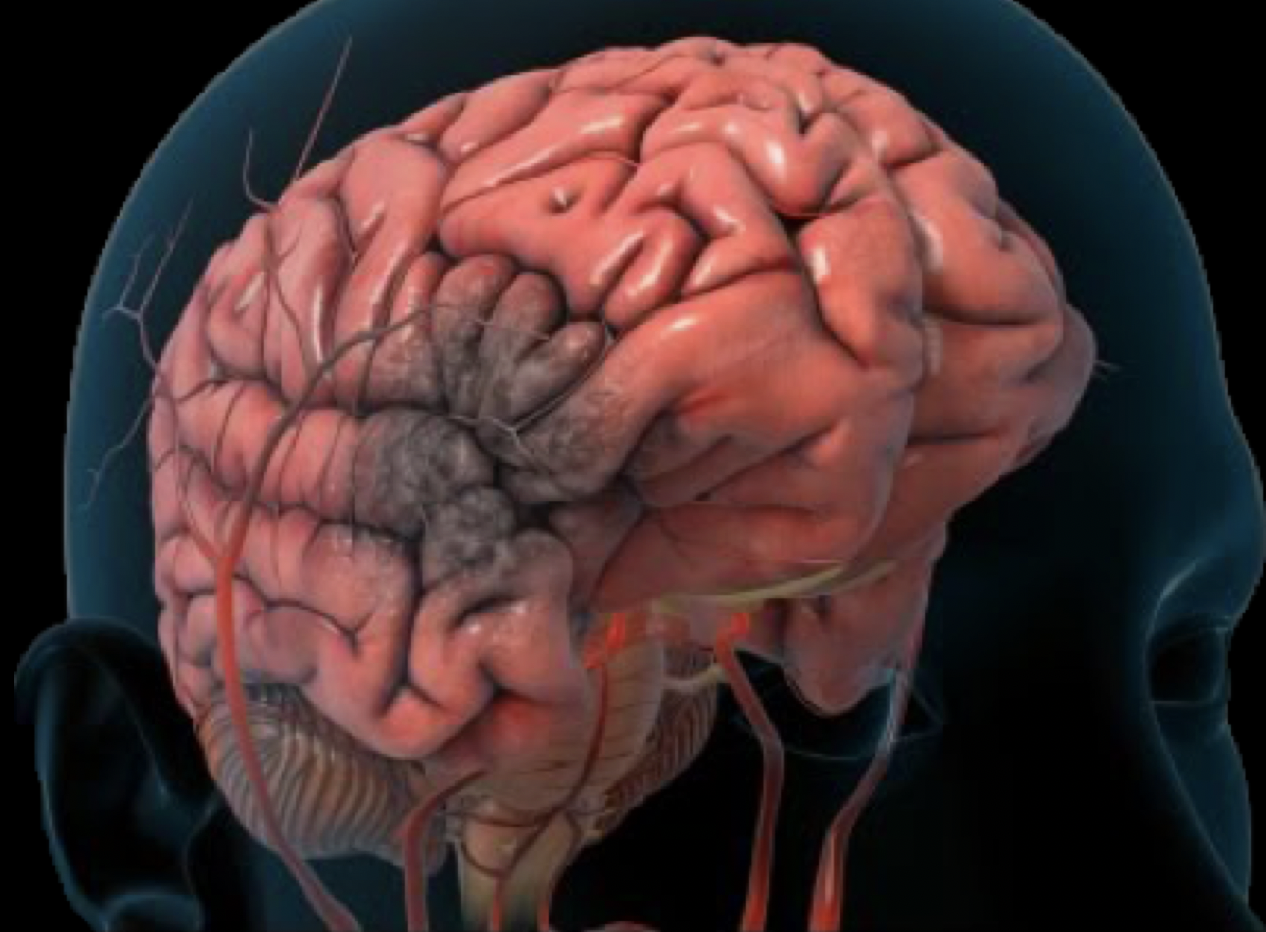

Current therapy for ischemic stroke is thrombolysis (tPA) and mechancal thrombectomy.
However, only 10% of all ischemic stroke patients (795,000 patients in US) currently undergo thrombectomy, which has become the standard of care since 2015. Of these patients, at best 40-50% are helped with this approach. Additionally, many are turned down for not having sufficient ‘salvageable’ brain tissue (penumbra).
No current therapy for TBI (concussion) except 'rest.'
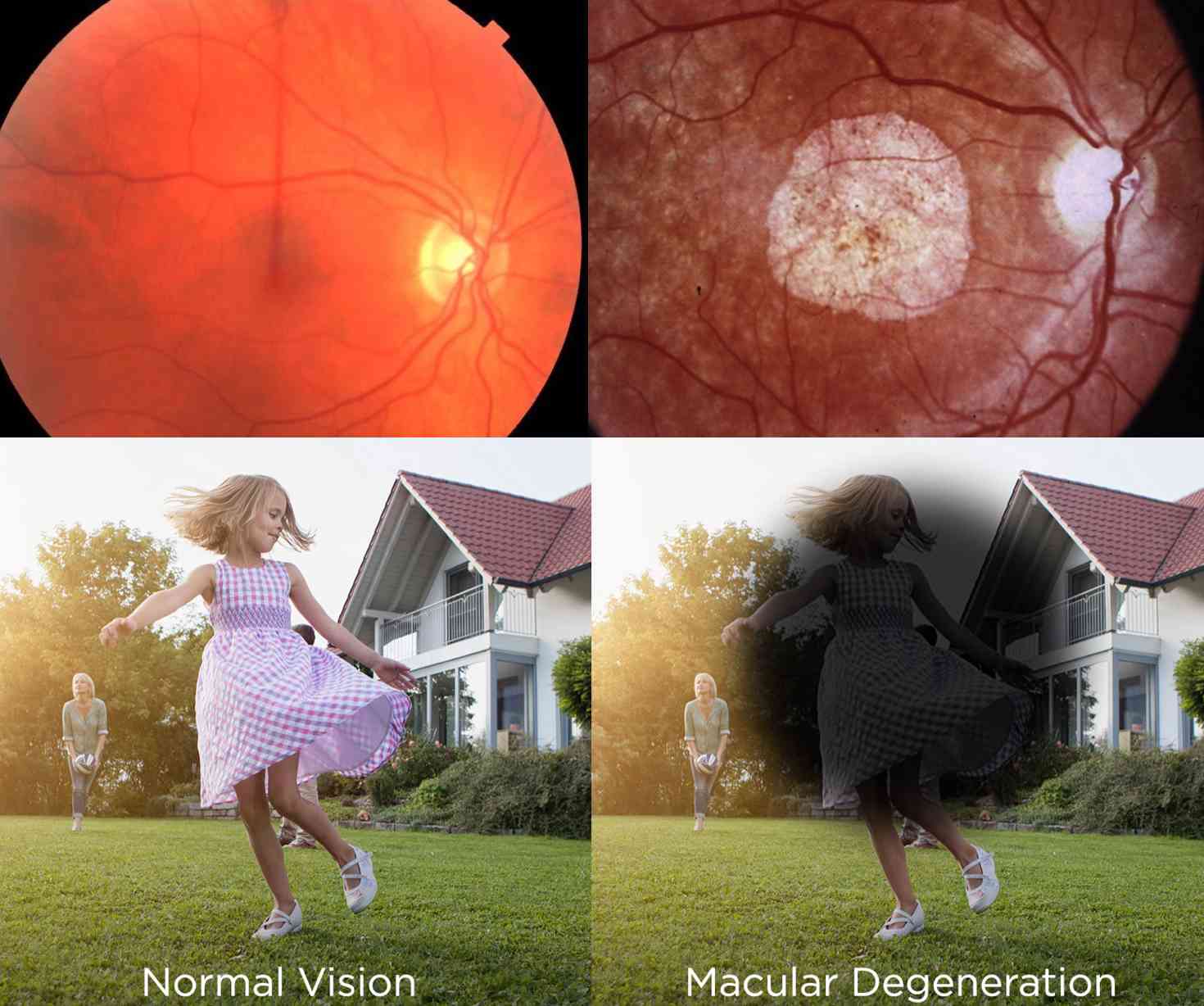
There is no current therapy that can halt or improve vision in dry AMD
Few effective options exists for patients with ischemic stroke and most do not receive it
Use of elovanoids for other neurodegenerative diseases with no current therapies, such as Alzheimer's, Parkinson's and other neurodegenerative diseases
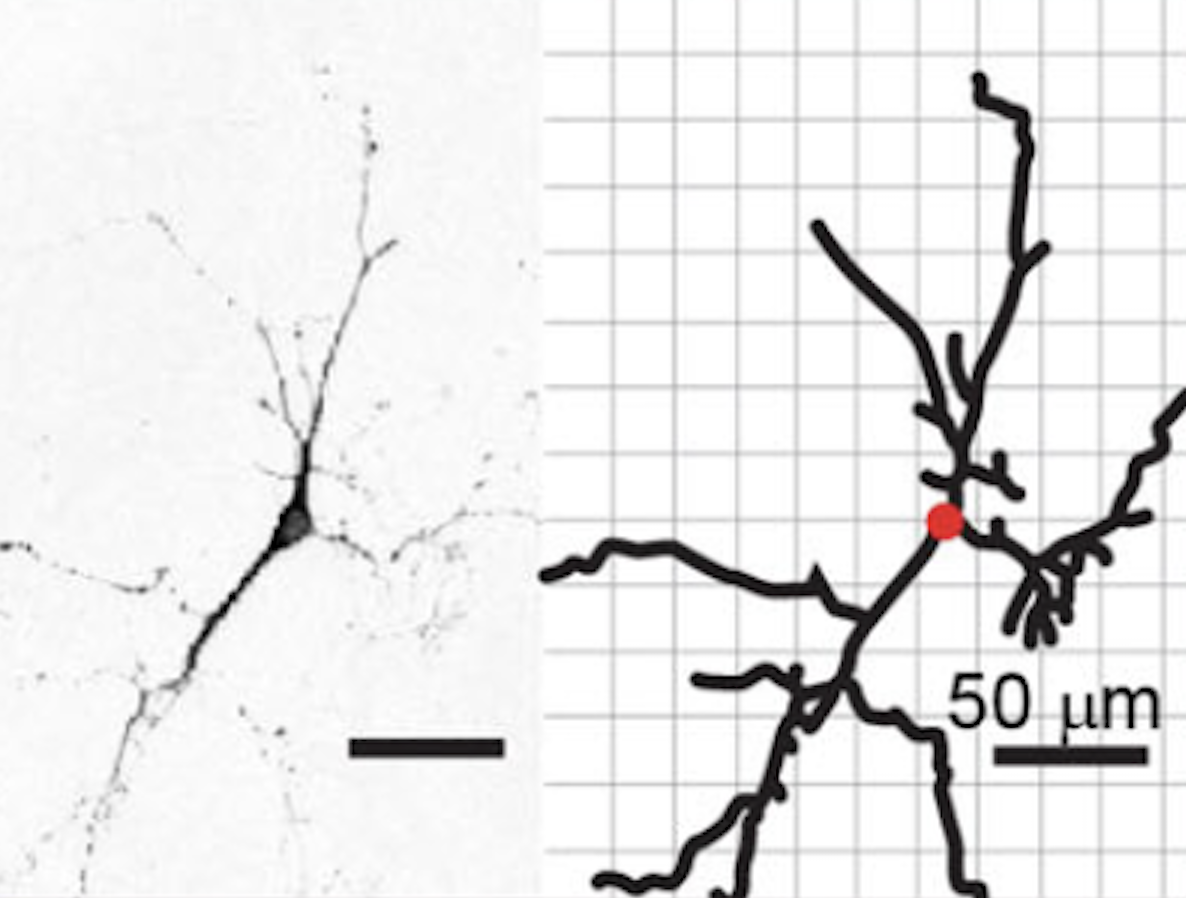
Despite decades of research, there is still no promising therapy for Alzheimer's
(Below, from our research group, left) Neurons in Alzheimer's disease and right) Degenerating dopaminergic-neurons of Parikinson's Disease)

AMD is #1 cause of blindness in Caucasians over the age of 65 in the USA (~11 million).
For dry AMD (85-90% of all AMD), there is no treatment except vitamins, which can only slow it down marginally – at best.
Currently, only neovascular ('wet') AMD can be treated, but not neurodegenerative ('dry') AMD, that affects the majority of AMD patients.
